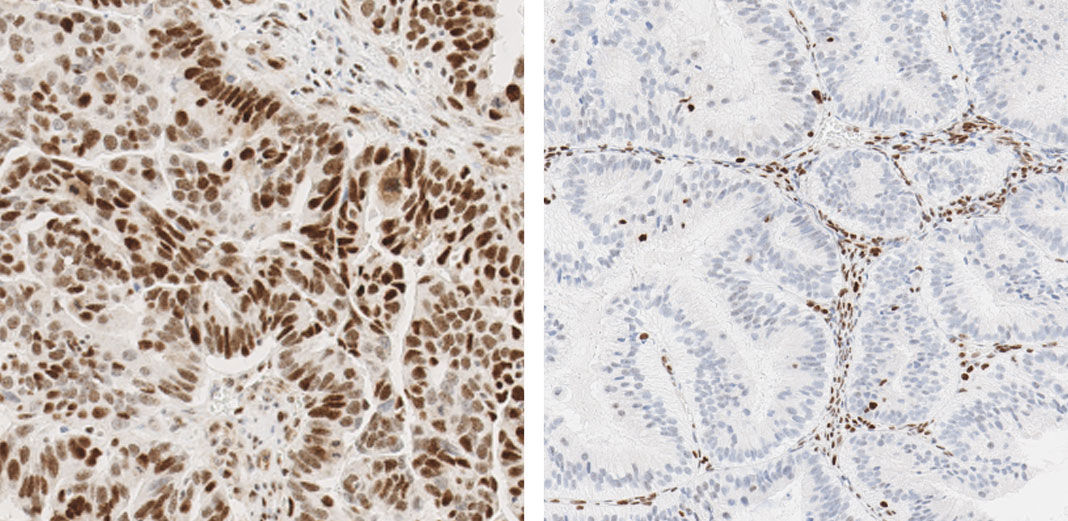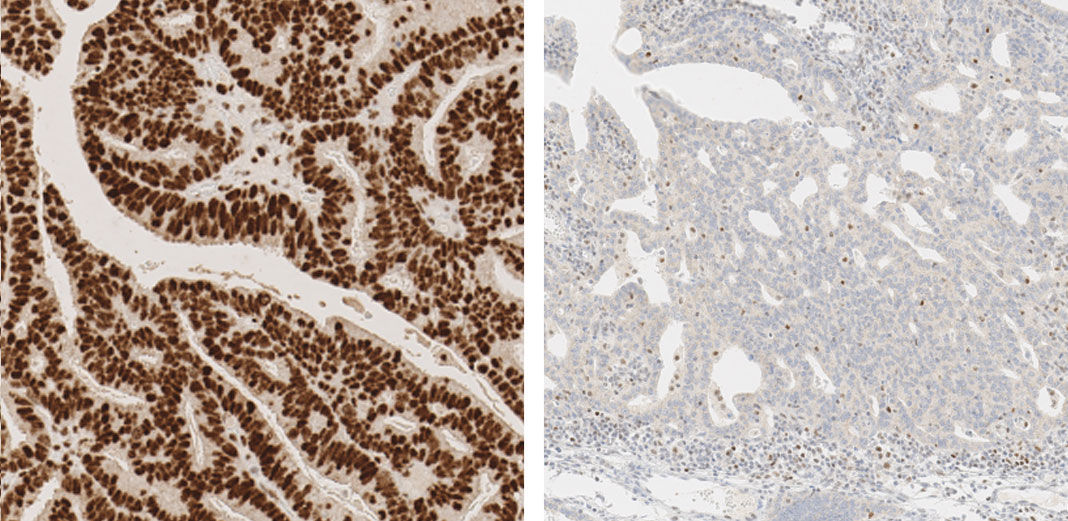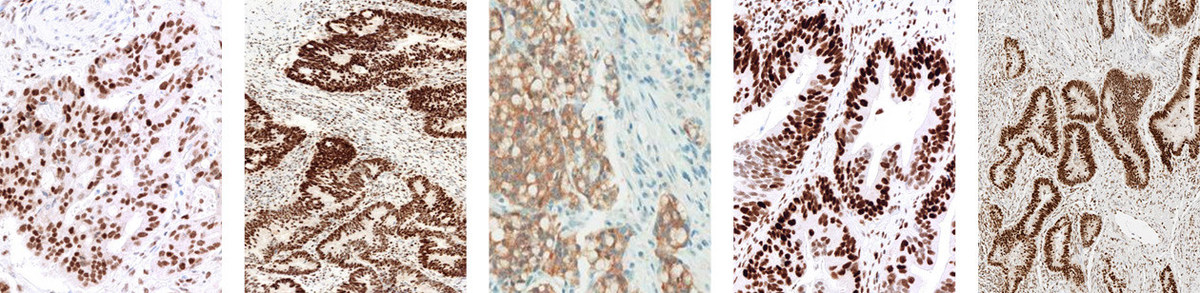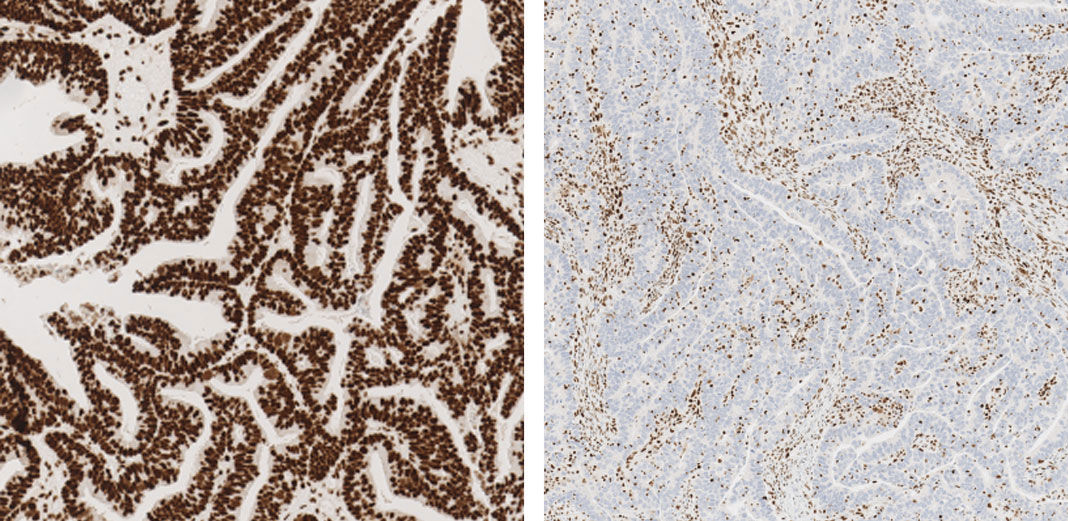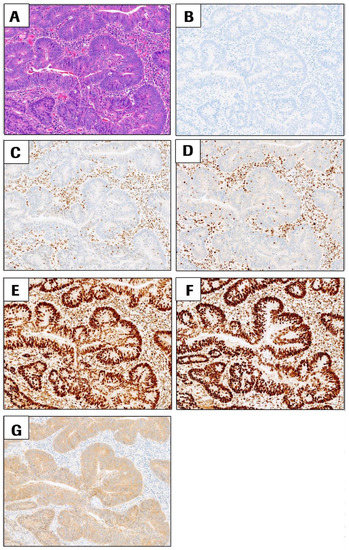
JMP | Free Full-Text | DNA Mismatch Repair Proteins and BRAF V600E Detection by Immunohistochemistry in Colorectal Cancer Demonstrates Concordance with Next Generation Sequencing
Ventana MMR IHC Panel DECISION SUMMARY A. De Novo Number: DEN170030 B. Purpose for Submission: De novo request for evaluation of

Roche Diagnostics USA on X: "Roche Diagnostics has received FDA approval for VENTANA MMR RxDx Panel to identify solid tumor patients eligible for JEMPERLI (dostarlimab-gxly) treatment. Learn more: https://t.co/enf2OnDbWw #roche #mmrrxdx #targetedtherapy

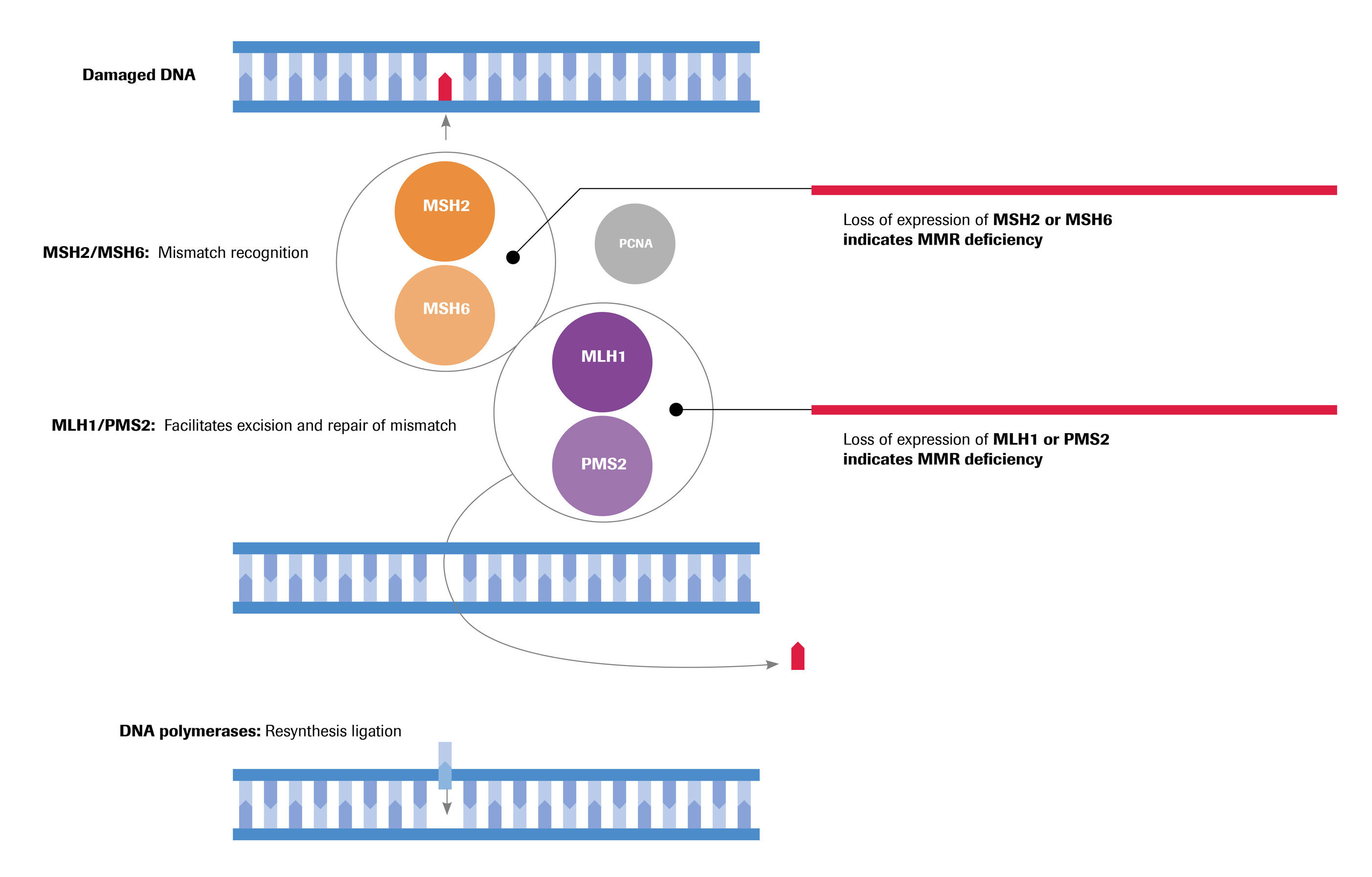
Detecting mismatch repair deficiency in solid neoplasms: immunohistochemistry, microsatellite instability, or both? - Modern Pathology

Roche Diagnostics USA on X: "It's time again for #TweetorialTuesday! This month we are focused on the VENTANA MMR IHC Colorectal Carcinoma Panel (VENTANA MMR IHC CRC Panel) #IHCPath #colorectalcarcinoma #mismatchrepair #chromosomalinstability #

Detecting mismatch repair deficiency in solid neoplasms: immunohistochemistry, microsatellite instability, or both? - Modern Pathology

Roche Receives FDA Approval for VENTANA MMR RxDx Panel to Identify dMMR Solid Tumour Patients and pMMR Endometrial Cancer Patients Eligible for KEYTRUDA - Medtech Alert
Ventana MMR IHC Panel DECISION SUMMARY A. De Novo Number: DEN170030 B. Purpose for Submission: De novo request for evaluation of
DNA Mismatch Repair Proteins and BRAF V600E Detection by Immunohistochemistry in Colorectal Cancer Demonstrates Concordance with